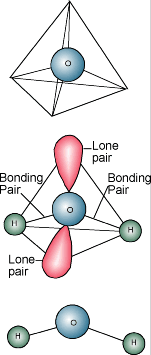
 You
probably know water's chemical description is H2O. A water molecule
consists of one atom of oxygen bound to two atoms of hydrogen. The hydrogen
atoms are "attached" to one side of the oxygen atom, resulting in
a water molecule having a positive charge on the side where the hydrogen atoms
are and a negative charge on the other side, where the oxygen atom is. Since
opposite electrical charges attract, water molecules tend to attract each other,
making water kind of "sticky." The side with the hydrogen atoms (positive
charge) attracts the oxygen side (negative charge) of a different water molecule.
You
probably know water's chemical description is H2O. A water molecule
consists of one atom of oxygen bound to two atoms of hydrogen. The hydrogen
atoms are "attached" to one side of the oxygen atom, resulting in
a water molecule having a positive charge on the side where the hydrogen atoms
are and a negative charge on the other side, where the oxygen atom is. Since
opposite electrical charges attract, water molecules tend to attract each other,
making water kind of "sticky." The side with the hydrogen atoms (positive
charge) attracts the oxygen side (negative charge) of a different water molecule.
All these water molecules attracting each other mean they tend to clump together. This is why water drops are, in fact, drops! If is wasn't for some of Earth's forces, such as gravity, a drop of water would be ball shaped -- a perfect sphere. Even if it doesn't form a perfect sphere on Earth, we should be happy water is sticky.
Water is called the "universal solvent" because it dissolves more substances than any other liquid. This means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients.
Pure water has a neutral pH. Pure water has a pH, of about 7, which is neither acidic nor basic.
Water's
Physical Properties:
Water is unique
in that it is the only natural substance that is found in all three states
-- liquid, solid (ice), and gas (steam) -- at the temperatures normally found
on Earth. Earth's water is constantly interacting, changing, and in movement.
 Water
freezes at 32° Fahrenheit (F) and boils at 212° F. In fact, water's freezing
and boiling points are the baseline with which temperature is measured: 0°
on the Celsius scale is water's freezing point, and 100° is water's boiling
point. Water is unusual in that the solid form, ice, is less dense than the
liquid form, which is why ice floats.
Water
freezes at 32° Fahrenheit (F) and boils at 212° F. In fact, water's freezing
and boiling points are the baseline with which temperature is measured: 0°
on the Celsius scale is water's freezing point, and 100° is water's boiling
point. Water is unusual in that the solid form, ice, is less dense than the
liquid form, which is why ice floats.
Water has a high specific heat index. This means that water can absorb a lot of heat before it begins to get hot. This is why water is valuable to industries and in your car's radiator as a coolant. The high specific heat index of water also helps regulate the rate at which air changes temperature, which is why the temperature change between seasons is gradual rather than sudden, especially near the oceans.
Water has a very high surface tension. In other words, water is sticky and elastic, and tends to clump together in drops rather than spread out in a thin film. Surface tension is responsible for capillary action, which allows water (and its dissolved substances) to move through the roots of plants and through the tiny blood vessels in our bodies.
Water
temperature:
Water temperature
is not only important to swimmers and fisherman, but also to industries and
even fish and algae. A lot of water is used for cooling purposes in power
plants that generate electricity. They need cool water to start with, and
they generally release warmer water back to the environment. The temperature
of the released water can affect downstream habitats. Temperature also can
affect the ability of water to hold oxygen as well as the ability of organisms
to resist certain pollutants.
pH:
pH is a measure
of how acidic/basic water is. The range goes from 0 - 14, with 7 being neutral.
pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates
a base. pH is really a measure of the relative amount of free hydrogen and
hydroxyl ions in the water. Water that has more free hydrogen ions is acidic,
whereas water that has more free hydroxyl ions is basic. Since pH can be affected
by chemicals in the water, pH is an important indicator of water that is changing
chemically. pH is reported in "logarithmic units," like the Richter
scale, which measures earthquakes. Each number represents a 10-fold change
in the acidity/basicness of the water. Water with a pH of 5 is ten times more
acidic than water having a pH of six.
Pollution can change a water's pH, which in turn can harm animals and plants living in the water. For instance, water coming out of an abandoned coal mine can have a pH of 2, which is very acidic and would definitely affect any fish crazy enough to try to live in it! By using the logarithm scale, this mine-drainage water would be 100,000 times more acidic than neutral water -- so stay out of abandoned mines.
Specific
Conductance:
Specific conductance
is a measure of the ability of water to conduct an electrical current. It
is highly dependent on the amount of dissolved solids (such as salt) in the
water. Pure water, such as distilled water, will have a very low specific
conductance, and sea water will have a high specific conductance. Rainwater
often dissolves airborne gasses and airborne dust while it is in the air,
and thus often has a higher specific conductance than distilled water. Specific
conductance is an important water-quality measurement because it gives a good
idea of the amount of dissolved material in the water.
Probably in school you've done the experiment where you hook up a battery to a light bulb and run two wires from the battery into a beaker of water. When the wires are put into a beaker of distilled water, the light will not light. But, the bulb does light up when the beaker contains salt water (saline). In the saline water, the salt has dissolved, releasing free electrons, and the water will conduct an electrical current.
Turbidity:
Turbidity
is a measure of the cloudiness of water. It is measured by passing a beam
of light through the water and seeing how much is reflected off particles
in the water. Water cloudiness is caused by material, such as dirt and residue
from leaves, that is suspended (floating) in the water. Crystal-clear water,
such as Lake Tahoe (where they work hard to keep sediment from washing into
the lake) has a very low turbidity. But look at a river after a storm -- it
is probably brown. You're seeing all of the suspended soil in the water. Lucky
for us, the materials that cause turbidity in our drinking water either settle
out or are filtered before the water arrives in our drinking glass at home.
Turbidity is measured in nephelometric turbidity units (NTU).
Dissolved
Oxygen:
Although water
molecules contain an oxygen atom, this oxygen is not what is needed by aquatic
organisms living in our natural waters. A small amount of oxygen, up to about
ten molecules of oxygen per million of water, is actually dissolved in water.
This dissolved oxygen is breathed by fish and zooplankton and is needed by
them to survive.
Rapidly moving water, such as in a mountain stream or large river, tends to contain a lot of dissolved oxygen, while stagnant water contains little. The process where bacteria in water helps organic matter, such as that which comes from a sewage-treatment plant, decay consumes oxygen. Thus, excess organic material in our lakes and rivers can cause an oxygen-deficient situation to occur. Aquatic life can have a hard time in stagnant water that has a lot of rotting, organic material in it, especially in summer, when dissolved-oxygen levels are at a seasonal low.
Hardness:
The amount
of dissolved calcium and magnesium in water determines its "hardness."
Water hardness varies throughout the United States. If you live in an area
where the water is "soft," then you may never have even heard of
water hardness. But, if you live in Florida, New Mexico, Arizona, Utah, Wyoming,
Nebraska, South Dakota, Iowa, Wisconsin, or Indiana, where the water is relatively
hard, you may notice that it is difficult to get a lather up when washing
your hands or clothes. And, industries in your area might have to spend money
to soften their water, as hard water can damage equipment. Hard water can
even shorten the life of fabrics and clothes! Does this mean that students
who live in areas with hard water keep up with the latest fashions since their
clothes wear out faster?
Suspended
Sediment:
Suspended
sediment is the amount of soil moving along in a stream. It is highly dependent
on the speed of the water flow, as fast-flowing water can pick up and suspend
more soil than calm water. During storms, soil is washed from the stream banks
into the stream. The amount that washes into a stream depends on the type
of land in the river's drainage basin and the vegetation surrounding the river.
If land is disturbed along a stream and protection measures are not taken, then excess sediment can harm the water quality of a stream. You've probably seen those short, plastic fences that builders put up on the edges of the property they are developing. These silt fences are supposed to trap sediment during a rainstorm and keep it from washing into a stream, as excess sediment can harm the creeks, rivers, lakes, and reservoirs.
Sediment coming into a reservoir is always a concern; once it enters it cannot get out - most of it will settle to the bottom. Reservoirs can "silt in" if too much sediment enters them. The volume of the reservoir is reduced, resulting in less area for boating, fishing, and recreation, as well as reducing the power-generation capability of the power plant in the dam.
Aqueous
Solution Geochemistry:
Look
at a diagram of the hydrogeochemical cycle.
- Acid = substance containing hydrogen which gives free hydrogen (H + ) when dissolved in water
- Base = substance containing the OH group that yields free (OH - ) when dissolved in water
-
An acid solution is one containing an excess of free H + , and a base is one containing excess of free OH - . A reaction between an acid and a base is usually called neutralization.
For example:
-
HCl (acid) + NaOH (base) ==> H 2 O + NaCl
which are dissociated into ions:
H + + Cl - + Na + + OH - ==> H 2 O + Na + + Cl - - i.e. Na + and Cl - are unaffected.
- pH = inverse log of the concentration (activity) of free H + , or pH = -log [H + ]
-
Water dissociates into H + and OH - ;
-
the dissociation constant is: K water = [H + ] [OH - ] =10 -14
- So there has to be 10 -7 moles each of H+ and OH - in a kilogram of neutral solution at standard temperature of 25°C. One mole is 6.023 x 10 23 atoms (or molecules) and H 2 O has a molecular weight of 18 grams per mole. One kilogram of water has about 1000/18 = 55.6 moles of water or about 3.35 x 10 25 atoms of oxygen and about twice that number (6.7 x 10 25 atoms) of H + (the amount of free H + or free OH - is relatively small compared to the amount of undissociated H 2 O).
- pH ranges at 25°C from 0 to 14; pH < 7 = acidic solution; pH > 7 = basic solution. If HCl or another acid is added then pH decreases; if NaOH or another base is added then pH increases.
- pH increases as carbonic acid (a weak acid) dissociates: When carbon dioxide combines with water, such as what happens in the atmosphere when fossil fuels are burned, carbonic acid is formed: H 2 O + CO 2 ==> H 2 CO 3 . Free H + are made available during successive dissociations:
-
H 2 CO 3 ==> H + + HCO 3- carbonic acid to bicarbonate, occurs at pH ~6.4
-
HCO 3 ==> H + + CO 32- bicarbonate to carbonate, occurs at pH ~10.3
Remember, free H + is available only when acidic, or when pH < ~7. The dissociation of bicarbonate to carbonate occurs when there is too much OH - in the system and H + is "released" to balance out the base.
- Dissolved Cations
and Anions in Water
Cations = electron donors, positively charged: Na + , K + , Mg ++ , Ca ++ , Fe ++ or Fe +++ , Mn ++ , Al +++
Anions = electron acceptors, neg. charged: Cl - , F - , I - , Br - , SO 4-- , CO 3-- , HCO 3- , NO 3-- , NO 2-
Metals = act like cations mostly: Cu, Zn, Pb, Co, Ni, Cr, As, Se, Mo, etc.
- Water Analyses - Need to have cation-anion balance
millequivalent (MEQ) = mole equivalent charge or anion or cation, measure of total charge due to the ion in question dissolved in the solution. Start with concentration, divide by mole wt., multiply by charge: XX mg/L / MW x CHG = MEQ
Example: NaCl in solution, Na = 50 mg/L (50 ppm): 50/23 x 1 = 2.17 MEQ
Cl = 77 mg/L (77 ppm): 77/35.5 x -1 = -2.17 MEQ
So, if the total cation and anion MEQ’s are not balanced, some error exists in the analysis.
Source Information