Atoms, Elements,
and Periodic Table
 Scientists
once believed that the atom was the most basic unit of matter; with the advent
of quantitative chemistry and new technologies enhancing our powers of observation
we found this to be untrue. Most chemistry textbooks detail the scientific inquiry
that lead to our current understanding of the elements and their physical properties.
This tutorial summarizes that work by explaining: 1) the electronic structure
of an atom and it’s components, 2) the effect of sequentially adding components
to an atom, 3) where the different types of components go, 4) what the affect
of adding the components has on the atom, (delta mass) 5) the significance of
the periodic arrangement of these components (delta electroneg), 6) how all this
knowledge was used to organize the periodic table, which may be viewed as a simple
spreadsheet.
Scientists
once believed that the atom was the most basic unit of matter; with the advent
of quantitative chemistry and new technologies enhancing our powers of observation
we found this to be untrue. Most chemistry textbooks detail the scientific inquiry
that lead to our current understanding of the elements and their physical properties.
This tutorial summarizes that work by explaining: 1) the electronic structure
of an atom and it’s components, 2) the effect of sequentially adding components
to an atom, 3) where the different types of components go, 4) what the affect
of adding the components has on the atom, (delta mass) 5) the significance of
the periodic arrangement of these components (delta electroneg), 6) how all this
knowledge was used to organize the periodic table, which may be viewed as a simple
spreadsheet.
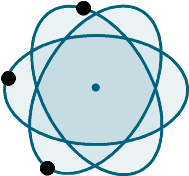
 A
neutral, or stable, atom is composed of an equal amount of three components:
protons, neutrons, and electrons. Protons have a positive electrical charge,
neutrons are neutral, and electrons have a negative charge. The most simple
atom to describe is the hydrogen atom, which has one proton and one neutron
that exist within the nucleus. The hydrogen atom also has an electron; which
balances the positive charge in the nucleus created by the proton. Elements
comprised of atoms having positive charges are the metals: alkali earth, and
transition metals. Scientists describe the physical behavior of these elements
as the metallic character; which is a comparison of each elements charge, size,
and mass. These three variables are generally set for each atom, but may vary
yielding two or three electronic states for a particular element (e.g. Iron
+2, and Iron +3). Hydrogen atoms, and all metals, seek out electrons to balance
that net positive charge. We will discuss the source later. The electron, once
found, orbits the nucleus at a distance that represents a balance between the
mass of the nucleus (protons + neutrons) and the velocity of the electron; which
is exactly like a satellite circling earth. The subsequent neutral orbit is
an orbital.
A
neutral, or stable, atom is composed of an equal amount of three components:
protons, neutrons, and electrons. Protons have a positive electrical charge,
neutrons are neutral, and electrons have a negative charge. The most simple
atom to describe is the hydrogen atom, which has one proton and one neutron
that exist within the nucleus. The hydrogen atom also has an electron; which
balances the positive charge in the nucleus created by the proton. Elements
comprised of atoms having positive charges are the metals: alkali earth, and
transition metals. Scientists describe the physical behavior of these elements
as the metallic character; which is a comparison of each elements charge, size,
and mass. These three variables are generally set for each atom, but may vary
yielding two or three electronic states for a particular element (e.g. Iron
+2, and Iron +3). Hydrogen atoms, and all metals, seek out electrons to balance
that net positive charge. We will discuss the source later. The electron, once
found, orbits the nucleus at a distance that represents a balance between the
mass of the nucleus (protons + neutrons) and the velocity of the electron; which
is exactly like a satellite circling earth. The subsequent neutral orbit is
an orbital.
The basic atom, which is
hydrogen, may become another element through fusion; which is combining two
hydrogen atoms to form one atom and some radioactive byproducts [nucleosynthesis].
During fusion between two hydrogen atoms, 1) one atom gains a proton, 2) the
other forms byproducts, 3) a new type of hydrogen (an isotope) called deuterium
forms which has two protons. Similar processes fusing hydrogen with other elements
sequentially form heavier elements; which have a resultant peroidic change in
mass. Chemists refer to this change as a change in mass, because a proton and
neutron have measurable masses and their addition to the nucleus makes heavier
atoms. Further, it is periodic because progressively heavier elements are created
by adding protons to different nuclei one at a time, sequentially starting with
the hydrogen atom. The second consequence of adding protons is that the net
charge of the nucleus also goes up, therefore as counterbalance electrons are
also added to the atom in new orbitals. Chemists reflected the resulting change
in mass by organizing the elements from lightest (1) to heaviest (178). However,
because adding protons and neutrons is balanced by electrons, changes in geometry
and electronegativity also occur.
Electrons are added to
the atom in a very periodic manner. Simply put, there is a pattern to it, and
the Aufbau Principle shows this pattern in a simple flow chart [AufBau]. The
orbitals, in which electrons reside, are pathways bounded by an outer shell
that is simply an outer limit in space for those orbitals. Four types of shells
exist: s, p, d, and f shells. Each shell has different amount of electron paths,
or an electron capacity. The capacities are: s = 2, p = 6, d = 10, and f = 12
electrons. The shells are filled sequentially, (with a slight twist) until thirty
electrons exist, then the shell sequence repeats itself again. The repetition
reflects the lowest energy state for each successive electron, the stable state
mentioned earlier. The shells in the first repetition are referred to as 2s,
2p, and so on, 3s, 3p, and so on in the third repetition. The Aufbau principle
allows us to map the addition the electrons, and understand that effect on atomic
geometry and electronegativity.
The changes in geometry
and electronegativity depend directly on the amount of electrons in the outer
shell. The geometry (size and shape) of an atom depends upon the mass of its
nucleus, which must increase with addition of protons, the subsequent amount
of electrons, and proportion of electrons in the outer shell. The mass to size
relationship of a nucleus should be self explanatory. One might assume that
because mass is added that size must also necessarily always increase too. But
this is not quite true The subsequent amount of electrons may vary about the
atom creating charged species (e.g. H+). The resulting species, shrink
when charge goes up by the loss of an electron, and expand when the net charge
goes down through gaining an electron. The transition metals exhibit this character
by having different oxidation states: e.g. Fe++, Fe+++.
An increase in the net charge causes the nucleus to exert more force on the
remaining electrons with in the outer shell, thus pulling it inward and shrinking
the outer limit of the atom. If the outer shell of the atom is full, then that
atom is electronegative. If the outer shell is not full, then the degree of
electronegativity depends upon which shell is partially filled and to what extent
it is filled.
The changes in electronegativity
depend directly on which type of shell is the outer shell and the amount of
electrons in the outer shell.
Species with net negative
charges may be considered electron acceptors, and net negative charges as electron
donors.
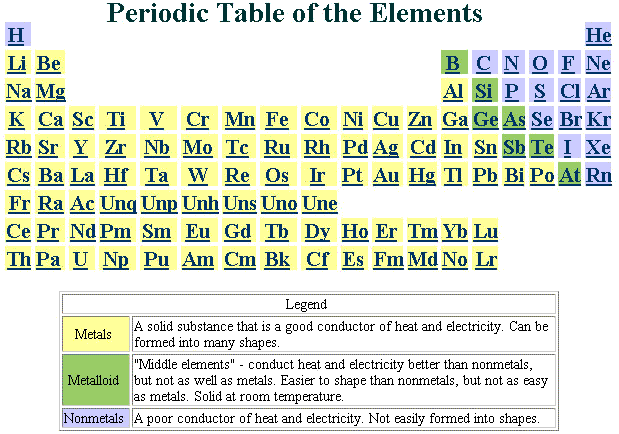
Look at an "unzipped"
periodic table.  A
neutral, or stable, atom is composed of an equal amount of three components:
protons, neutrons, and electrons. Protons have a positive electrical charge,
neutrons are neutral, and electrons have a negative charge. The most simple
atom to describe is the hydrogen atom, which has one proton and one neutron
that exist within the nucleus. The hydrogen atom also has an electron; which
balances the positive charge in the nucleus created by the proton. Elements
comprised of atoms having positive charges are the metals: alkali earth, and
transition metals. Scientists describe the physical behavior of these elements
as the metallic character; which is a comparison of each elements charge, size,
and mass. These three variables are generally set for each atom, but may vary
yielding two or three electronic states for a particular element (e.g. Iron
+2, and Iron +3). Hydrogen atoms, and all metals, seek out electrons to balance
that net positive charge. We will discuss the source later. The electron, once
found, orbits the nucleus at a distance that represents a balance between the
mass of the nucleus (protons + neutrons) and the velocity of the electron; which
is exactly like a satellite circling earth. The subsequent neutral orbit is
an orbital.
A
neutral, or stable, atom is composed of an equal amount of three components:
protons, neutrons, and electrons. Protons have a positive electrical charge,
neutrons are neutral, and electrons have a negative charge. The most simple
atom to describe is the hydrogen atom, which has one proton and one neutron
that exist within the nucleus. The hydrogen atom also has an electron; which
balances the positive charge in the nucleus created by the proton. Elements
comprised of atoms having positive charges are the metals: alkali earth, and
transition metals. Scientists describe the physical behavior of these elements
as the metallic character; which is a comparison of each elements charge, size,
and mass. These three variables are generally set for each atom, but may vary
yielding two or three electronic states for a particular element (e.g. Iron
+2, and Iron +3). Hydrogen atoms, and all metals, seek out electrons to balance
that net positive charge. We will discuss the source later. The electron, once
found, orbits the nucleus at a distance that represents a balance between the
mass of the nucleus (protons + neutrons) and the velocity of the electron; which
is exactly like a satellite circling earth. The subsequent neutral orbit is
an orbital.  Scientists
once believed that the atom was the most basic unit of matter; with the advent
of quantitative chemistry and new technologies enhancing our powers of observation
we found this to be untrue. Most chemistry textbooks detail the scientific inquiry
that lead to our current understanding of the elements and their physical properties.
This tutorial summarizes that work by explaining: 1) the electronic structure
of an atom and it’s components, 2) the effect of sequentially adding components
to an atom, 3) where the different types of components go, 4) what the affect
of adding the components has on the atom, (delta mass) 5) the significance of
the periodic arrangement of these components (delta electroneg), 6) how all this
knowledge was used to organize the periodic table, which may be viewed as a simple
spreadsheet.
Scientists
once believed that the atom was the most basic unit of matter; with the advent
of quantitative chemistry and new technologies enhancing our powers of observation
we found this to be untrue. Most chemistry textbooks detail the scientific inquiry
that lead to our current understanding of the elements and their physical properties.
This tutorial summarizes that work by explaining: 1) the electronic structure
of an atom and it’s components, 2) the effect of sequentially adding components
to an atom, 3) where the different types of components go, 4) what the affect
of adding the components has on the atom, (delta mass) 5) the significance of
the periodic arrangement of these components (delta electroneg), 6) how all this
knowledge was used to organize the periodic table, which may be viewed as a simple
spreadsheet.