What is
Radiation?
Radiation
is everywhere. It is a form of energy. Radiation is produced when the nucleus
of an atom is broken apart. Huge amounts of energy are stored in the nucleus.
When it splits (called fission) it produces heat and radiation. Nuclear
power plants use the heat to turn water into steam and produce electricity.
The radiation, and any radioactive materials generated are considered to be
waste products. More than four-fifths of the radiation we receive comes from
natural sources. This is called background radiation because it is present everywhere,
all the time. Radioactive atoms are often called radionuclides.
Radioactive
Decay & Half-Life
When atoms
split apart naturally it is called decay. Decay happens because
certain elements have unstable nuclei - a situation occurring because
they have a whole lot of neutrons and so the nuclear forces are not balanced.
 When an atom decays it
can form a different isotope of the same element (i.e. it loses neutrons) or
it can become a new element (i.e. it loses protons). It is important to realize
that, just because atoms have experienced one decay, it does not mean that they
are no longer radioactive. Some elements decay multiple times and go through
a series of elements that are all radioactive. Eventually though, enough
time will pass and a stable elemental form will be reached. An example
of this is the decay series of which Radon Gas is an intermediate. This
series has multiple decay steps from the original Uranium atoms to final, stable
lead isotopes.
When an atom decays it
can form a different isotope of the same element (i.e. it loses neutrons) or
it can become a new element (i.e. it loses protons). It is important to realize
that, just because atoms have experienced one decay, it does not mean that they
are no longer radioactive. Some elements decay multiple times and go through
a series of elements that are all radioactive. Eventually though, enough
time will pass and a stable elemental form will be reached. An example
of this is the decay series of which Radon Gas is an intermediate. This
series has multiple decay steps from the original Uranium atoms to final, stable
lead isotopes.
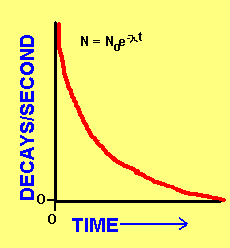
One important
property of radioactive elements is half-life. Half-life is defined
as the amount of time it takes for one half of all of the atoms of that element
to experience a decay. Because you are halving the number of atoms each
time, the amount of atoms of a particular element (and so the total number of
decays) decrease exponentially! N is the number of decays/second (or atoms)
at a particular time t. No is the initial amount. Lambda is a decay constant.
As
an example, lets say you start with 1000 atoms of a radioactive element that
has a half-life of 10 years. In 10 years you will have 500 atoms; in 20
years you will have 250 atoms; in 30 years you will have 125 atoms and so on...until
they have all decayed.
Half-life
is an extremely important property of radiation. It is used to determine
how long a particular radioactive species will remain radioactive. That
is, it tells how long that species may present a danger to human health and
the environment. Half-life varies widely from element to element.
For example Uranium-235 has a half-life of 704 million years while Oxygen-9
has a half-life of only 26.9 seconds! Half-life is an important consideration
when attempting to determine how to dispose of a particular radioactive waste.
This is because the waste must be contained for as much time as it takes to
render it harmless. In general, a radioactive species is considered to
be harmless when it reaches background levels - usually after about 10 half-lives.
Sources
of Radiation
Radiation
is a natural part of our every day lives. There are all kinds of sources of
radiation. Some of these are:
- RADON
GAS -this is a significant natural source.
- COSMIC
RAYS -can act on nuclei in the atmosphere to produce radionuclides.
- COMBUSTION
OF FOSSIL FUELS -fly ash, produced during the combustion of coal,
contains a significant amount of radionuclides, even more than an equivalent
nuclear power plant!
- NUCLEAR
REACTORS / REPROCESSING SPENT FUEL -this is a significant source
of Krypton-85 in the atmosphere.
- NUCLEAR
WEAPONS -the above ground detonation of nuclear weapons releases
tremendous amounts of radionuclides into the atmosphere.
In addition,
people are exposed to radiation from manufactured sources such as color televisions
and smoke detectors.
Types
of Radiation
Radiation can
occur either as high speed particles or as waves. Non-ionizing radiation
is low energy radiation and cannot alter atoms. Examples of non-ionizing radiation
are microwaves, infrared, and visible light. Ionizing radiation is high energy
radiation that is capable of altering atoms.
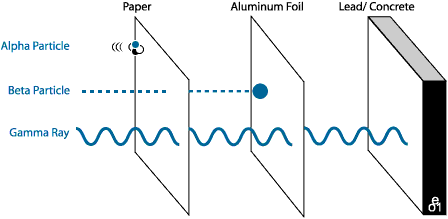
ALPHA
PARTICLES - the slowest, least energetic type. They do not travel
far, and can be blocked by almost anything. In general alpha particles cause
damage only if inhaled or swallowed.
BETA
PARTICLES - are more energetic. Beta
particles can pass through cloth or paper, but can be stopped by a sheet of
foil, or glass. Like alpha particles, beta particles generally are harmful only
if inhaled or swallowed. For example, Radon gas emits beta particles, and is
harmful because it is inhaled.
GAMMA
RAYS - are the most energetic, and dangerous. These are rays, not
particles and they can pass through almost anything. It takes very, very thick
concrete, lead or steel to block gamma rays. This type of radiation is dangerous
and damaging.
Each
radioactive element emits its own characteristic form of radiation.
Types
of Radioactive Waste:
LOW
LEVEL WASTES - do not present a significant hazard. They emit
low levels of radiation, usually in the form of alpha particles, and generally
have short half-lives. These wastes tend to be suitable for burial given
a 500 year containment. Some examples of low level waste are solution
residuals from processing operations, contaminated equipment, contaminated clothing
etc. The historically preferred method for disposing of low level waste
s was dilute and disperse. This is no longer an acceptable practice.
HIGH
LEVEL WASTES - These wastes are very radioactive and present a real
threat to human health and the environment. They tend to be beta or gamma (or
both) emitters. High level wastes are problematic to store, contain and dispose
of. Examples of high level wastes are the fission byproducts in spent
fuel rods and fuel assemblies from nuclear reactors such as Krypton-85 (gamma
and beta emitter;half-life=10.72 years), Strontium-90 (beta emitter;half-life=29.1
years) and Cesium-137 (gamma emitter;half-life=30.17 years).
TRANSURANIC
WASTE - Transuranic wastes can be low level, high level or both,
and consist of radioactive materials with atomic numbers greater than 92 (they
have greater than 92 protons).
Another type of radioactive
waste is MIXED WASTE which is a combination of radioactive and
hazardous waste (for regulatory purposes these are taken as two different things
SOURCE
- U, Th, or any other material that is determined by the AEC to be a significant
source of radioactivity. In particular, they regulate ores containing
U, Th or other radioactive species in concentrations high enough to be useful
for the manufacture of nuclear power or nuclear weapons
SPECIAL
- any radioactive material that the AEC designates as significant enough to
regulate, but that is not source material.
BYPRODUCT
- any other naturally radioactive material , or material made radioactive via
exposure to a radiation incident produced during such processes as the manufacturing
of weapons, reactors, mining source material etc.
Illustrations by Diane Boyak
and Ean Harker,© 2000-01
 When an atom decays it
can form a different isotope of the same element (i.e. it loses neutrons) or
it can become a new element (i.e. it loses protons). It is important to realize
that, just because atoms have experienced one decay, it does not mean that they
are no longer radioactive. Some elements decay multiple times and go through
a series of elements that are all radioactive. Eventually though, enough
time will pass and a stable elemental form will be reached. An example
of this is the decay series of which Radon Gas is an intermediate. This
series has multiple decay steps from the original Uranium atoms to final, stable
lead isotopes.
When an atom decays it
can form a different isotope of the same element (i.e. it loses neutrons) or
it can become a new element (i.e. it loses protons). It is important to realize
that, just because atoms have experienced one decay, it does not mean that they
are no longer radioactive. Some elements decay multiple times and go through
a series of elements that are all radioactive. Eventually though, enough
time will pass and a stable elemental form will be reached. An example
of this is the decay series of which Radon Gas is an intermediate. This
series has multiple decay steps from the original Uranium atoms to final, stable
lead isotopes. 